About Us
Fillauer Motion Control
Fillauer’s mission is to lead the evolution, design, and technology of orthotic and prosthetic products and services that empower clinicians and patients to achieve their best functional outcomes.

Paving the way toward natural motion
COMPANY INFORMATION
Fillauer Motion Control is now entering our second century as a global leader in the orthotics and prosthetics industry! Today, we are still dedicated to providing comfortable solutions that enhance the lives of our patients and partners.
“Fillauer has been a leader in the evolution,
design and technology of orthotic
and prosthetic products and services.”
History
Motion Control, Inc., is one of the most advanced development and manufacturing companies in the prosthetics industry.
1974
Motion Control was established in 1974 by Dr. Stephen C. Jacobsen, to commercialize the medical technology developed at the University of Utah’s Center for Engineering Design. President Emeritus, Harold H. Sears, Ph.D., is an alumnus of Dr. Jacobsen’s team. In 2017, Arthur D. Dyck was named President of Motion Control.
 1981
1981
Since it was first available in 1981, the Utah Artificial Arm has been at the forefront of myoelectric technology for elbow, hand, and wrist. For over three decades Motion Control has developed cutting-edge improvements in electronics, and innovative design for function, natural appearance, and comfort.
1997
In 1997, the Utah Arm 2 (U2) brought a new level of ruggedness and dependability through improvements in electronics, battery power, and motor technologies.
Motion Control was acquired by Fillauer Companies, Inc. in January 1997, and became part of one of the most comprehensive orthotic and prosthetic companies in the world. Fillauer is headquartered in Chattanooga, Tennessee, and Motion Control operates in Salt Lake City, Utah.
1999
The ProControl 2, released in 1999, represented the first widely-distributed microprocessor to control the hand and wrist, with a computer interface and unique AutoCal feature. Motion Control also manufactures the Myolab II EMG Tester, a portable and convenient tool for testing and training patients being fitted with a myoelectric prosthesis.
 2002
2002
In 2002, Motion Control released the Motion Control (MC) Hand, and soon after, the Electric Terminal Device (ETD) to broaden the function of myoelectric products for the upper extremity amputee. When combined with the MC Flexion and Multi-Flex Wrists (and introduced in 2006, the ProWrist Rotator) the MC Hand and ETD are an excellent combination of function and cosmesis. Both the MC Hand and ETD are available with an onboard microprocessor (ProPlus version) so they may be used with other manufacturers’ systems.
2004
In 2004, microprocessor technology was incorporated into the Utah Arm 3 (U3), allowing separate inputs and simultaneous control of both, and a computer interface simplifying elbow and hand controls. Many veterans of recent military conflicts have been fitted with the Utah Arm 3 at Walter Reed National Military Medical Center, and San Antonio Military Medical Center.
2008-2009
The Utah Arm 3+ (U3+) added a two-stage lock and silent freeswing features in 2008.
The most innovative new foot/ankle system of recent years, Motionfoot® was introduced in 2009 as the only hydraulic foot/ankle to offer a normal range of ankle motion, coupled with the energy return of a carbon graphite sole-plate element. Enhancements to the design and durability of the Motionfoot® resulted in the Motionfoot®MX, released in 2014.
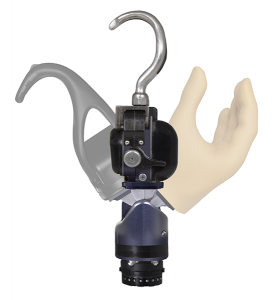 2017
2017
In 2017, a completely redesigned Electric Terminal Device hook was released as the ETD2. With its shorter length, field-replaceable gripping pads, outside friction surfaces, wide-opening fingers and streamlined design, the ETD2 continues the tradition of excellence expected from Motion Control products.
That same year, Motion Control became the manufacturer and distributor of the former Hosmer E2 Elbow, renamed the Motion E2 Elbow, offering a lower-cost option for switch-operated and myoelectric elbows, as Motion Control continues to develop new and innovative products for the prosthetic industry.
In late 2017, Motion Control became the exclusive U.S. distributor of the world’s first heavy duty, water resistant, multi-articulating hand, the Taska® Hand, expanding their line of heavy-duty upper limb prostheses.
2021
Motion Control’s products are sold exclusively to trained prosthetists and professionals in the orthotic and prosthetic industry to ensure a high standard of quality patient care. Products are sold worldwide, but mainly in the United States and Europe. Motion Control follows FDA and ISO quality control standards.
Management Team:
- President: Arthur D. Dyck, EET
- President Emeritus: Harold H. Sears, PhD
- Vice President of Research and Development: Edwin K. Iversen, MEME
- Director of Clinical Education: R. Scott Hosie, CPO
